Making fuel from solar energy is the holy grail of the renewable industry. In 2008, David Nocera, an energy professor from MIT, gushed about a “major discovery” which would unleash a solar revolution. The MIT press reported on a “simple, inexpensive, highly efficient process for storing solar energy”. Ostensibly this was a way to split water to store solar energy in the form of hydrogen and each house would have its own solar panel, which would mean that “electricity-by-wire from a central source could be a thing of the past”. These fairly ludicrous assertions are debunked here, however this did not stop one news outlet proclaiming that this invention was “why oil really fell today – and could keep falling”.
It was with this story in mind that I read of Caltech Professor Sossina Haile’s claim in Science a few days ago to have tested a new way to produce a solar fuel which would “make a major contribution to global gasoline supplies” and was not “cost prohibitive” either.
This method uses concentrated sunlight to heat up microporous CeO2 (ceria) to 1600 celsius. The elevated temperature drives out the oxygen to leave Ce. An inert gas is subsequently pumped through the chamber to remove the oxygen. The cerium is then cooled down to 900C. H2O and CO2 are pumped in. The relatively cool cerium strips the oxygen from the water and carbon dioxide to leave a syngas of H2 and CO. This can be the feedstock to a Fischer-Tropsch or Mobil process plant to make diesel or gasoline.
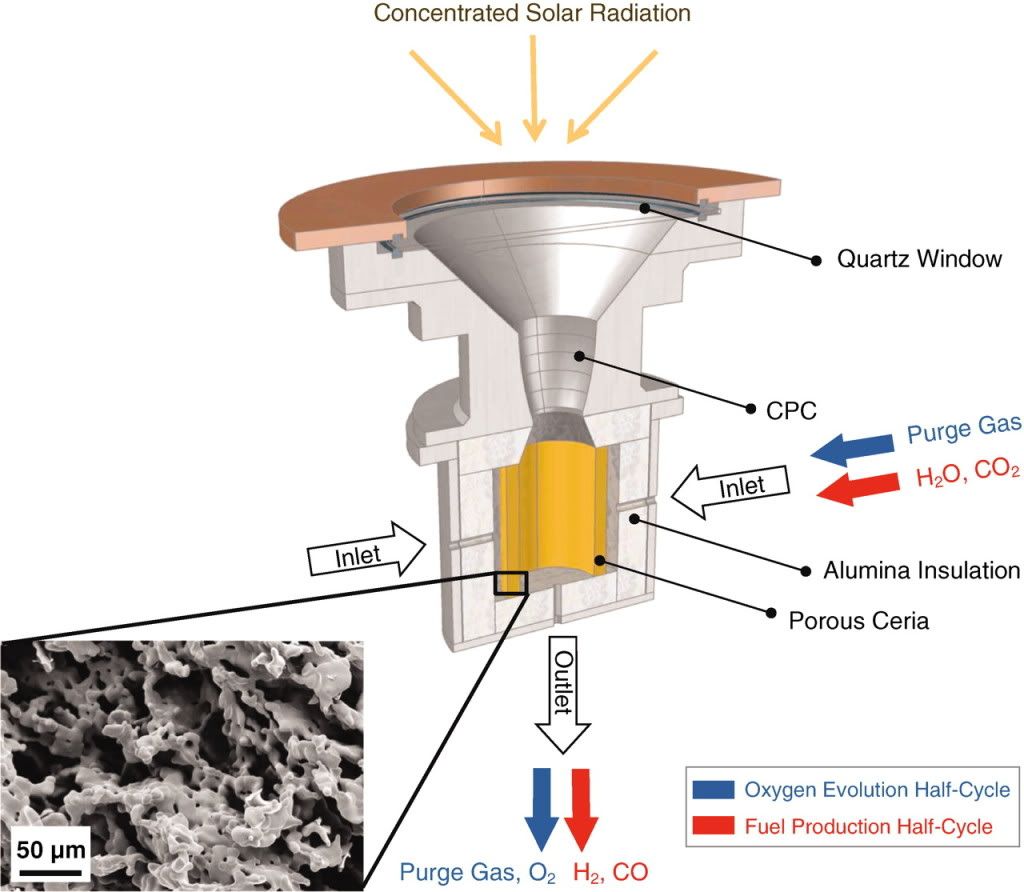
The experiment was obviously lab scale, with an area of a few cm^2. The radiation was artificial, but was at an intensity of 1500 suns (1 sun = 1kW/m^2) to generate the high temperatures, which is allegedly typical of solar tower set-ups. The 11MWe PS10 tower in Spain, for example, only has an intensity of 645 suns, but I won’t quibble with that. The achieved efficiency in this experiment was 0.8%, however this assumed no energy cost for the pure CO2, the H2O, the purge argon gas or the embodied energy of the setup, but not to worry. It is suggested that the CO2 would come from a co-located CCGT. Where the water would come from in a desert location is not clear.
The paper states that with refinement the efficiency (narrowly defined) can approach 20%. This happens to be the same efficiency (electrical output as % of reflected solar energy) as the PS10. So conceivably this setup could generate syngas at the same cost as the electricity from the PS10. The PS10 cost €3m/MW and the electrical output has a levelised cost of about 25€cent/kWh. This translates to approximately $100/MMBtu. The PS10 outputs about 20GWh per year and operates at a 20% capacity factor. This setup would provide enough energy for a rather pathetic 33 barrels of oil per day. Assuming a 400MW solar site, this gets the output up to about 1kb/d. Gas to liquid plants are only economic at large scales in the region of 30kb/d (Sasol last week stumped up $1bn to buy some Canadian shale and is exploring building a 40kb/d plant which it estimates can produce a high quality gallon of diesel for $1.50). So faced with natural gas at a current input price of $4/MMbtu (although slightly higher due to energy loss in the gasifier), an unfeasibly large required solar site, and not even mentioning the intermittency of output, I think we can safely put this idea to bed.
So while this is very interesting science, I wish scientists could be reticent in claiming to have found solutions to the energy fix since it can be counter-productive if it leads to complacency. Perhaps I could have shortened this article by simply stating Upton Sinclair’s insightful observation “It is difficult to get a man to understand something, when his salary depends upon his not understanding it!”



Careful there Eamon, you’re beginning to sound like me.